What is Catalysis?
It is the process of enhancing the pace of the reaction by introducing compounds known as catalysts. Catalysts are not utilised in the reactions and hence remain unaffected. If the response is the catalyst and recycles faster. The rate of reaction is affected by the surface area of the mixture and the temperature. Catalysts often react with one or more reactants to generate an intermediate that then reacts with the end reactant product, renewing the catalyst in the process.
Video 1: Synthetic wastewater catalyzed reaction in the presence of hydrogen peroxide.
Table 1: Catalyst usage and use in chemical industries through several processes.
Types of catalyst
Catalyst is classified into two categories namely,
- Homogenous Catalyst: In the chemical reactions where the reactants involved in the reaction and the catalyst are in the same phase.
- Heterogeneous Catalyst: In the chemical reaction where the reactant involved in the reaction and the catalyst are in different phases.
INDUSTRIAL CATALYSIS TODAY
Chemical industries that produce industrial catalysts have some general steps they follow for manufacturing these catalysts. Some of these processes are:
- Choosing the right ingredient suitable for the production of the catalyst
- Mixing these ingredients (in solution or suspensions)
- Crystallization
- Filtration
- Washing
- Mixing and kneading powders
- Drying
- Mixing with a forming agent, lubricant, and binder
- Shape formation
- Impregnation
- Calcination
- Activation or reduction to the preferred oxidation state
Methanol Production
Methanol is one of the largest production rates in industries for carbonylation products. The process uses syngas as feedstock. As a result, the water gas shift reaction is critical for this synthesis. The most significant methanol reaction is the breakdown of methanol to produce carbon monoxide and hydrogen. Methanol is therefore a significant raw ingredient for the creation of CO and H2, which may be utilised to generate fuel.
Nowadays, synthesis gas is often produced by steam reforming natural gas. Cu, Ni, Pd, and Pt are the most effective methanol synthesis catalysts, whereas Al and Si are the most commonly employed support metals. ICI (Imperial Chemical Industries) invented a technique that is still in use today in 1966. The low-pressure method employs a Cu/ZnO/Al2O3 catalyst, with copper serving as the active ingredient. This catalyst is the same as the low-temperature shift catalyst used in the WGS process.
A reaction mechanism for methanol synthesis has been suggested by Chinchen et al. HydroDesulfurization process of Crude oil
HDS catalysts are materials having a large surface area. When the reaction happens in a slurry reactor, they offer a large interfacial area between the liquid and gaseous phases. They include an active component as well as an accelerator that is spread on a base (alumina). Cobalt–molybdenum and nickel–molybdenum supported on alumina are the major catalysts.
Alumina offers excellent textural and mechanical qualities and is reasonably inexpensive. The Alumina support is primarily utilised to improve catalyst dispersion, which in turn improves catalytic performance. Cobalt-molybdenum catalyst has a modest hydrogenation activity and hence consumes less hydrogen for sulphur removal.
Mechanisms
- Introduction and diffusion of reactant molecules on the catalytic surfaces.
- Adsorption of molecules of reactants of catalytic surfaces.
- Formation of intermediate of the catalytic surface by a chemical reaction b/w reactant molecules.
- Desorption of product of molecules from the catalyst.
- Diffusion of product molecules away from the catalytic surface to form the final product.
Photocatalysis
Figure 2: Photocatalysis process mechanisms
Photocatalysis is the activity occurring when a light source interacts with the surface of semiconductor materials, called photocatalysis. It is the amalgamation of photochemistry and catalysis. Basically "photo" means "light" and catalysis means "increases the rate of the reaction".
The basic principle is that the organic molecules come into contact with the surface of the photocatalyst under UV light irradiation which leads to occur a series of oxidation and reduction reactions. during this process, there must be at least two simultaneous reactions from photogenerated holes, and reduction from photogenerated electrons. The generation of reactive hydroxyl radicals for the degradation of recalcitrant compounds.
When the light incidents on the surface catalyst. the catalyst absorbs light at a specific wavelength with the corresponding promotion of an electron from the valence band to the conduction band which leaves a hole in the valence band after moving of electron from the VB to CB. This can be represented as:
Catalyst + hv = e ^-(cb) (Catalyst) + h ^ (+vb) (catalyst), where, CB is the conduction band and vb is the VB.
The holes would be left in the VB of the catalyst. These holes in the VB can oxidise donor molecules and react with water molecules to generate hydroxyl radicals (The hydroxyl radicals' oxidizing power is responsible for the generation of pollutants.
A reaction takes place between electrons from the CB and Dissolved oxygen species to form superoxide ions in which these electrons propel the redox reactions.
Photocatalysis = h+ + (e-)
h+ + H2O = OH + (h+)
h+ + OH- = OH
h+ + pollutant = pollutant -
e- + O2 = O2
O2 + H+ = OOH
2*OOH = O2 + H2O2
H2O2 + O2 = OH + OH- + O2
H2O2 + hv = 2*OH
pollutant + (OH, H+, *OOH or O2-) = pollutant degradation
Applications:
Removal of heavy metals, Degradation of toxic pollutants, Degradation of specific contaminants, Sludge treatment, Water splitting, Removal o harmful gases, No disinfection by-products, Metal reduction, Building exterior self-cleaning, Reduction of colour and odour, removal of pollution. Sterilization.
Different Types of Photocatalysis
Titanium Dioxide, Zinc Oxide, Magnesium Oxide, tungsten Oxide, Iron Oxide or ferric oxide, Silicon Carbide, Gallium Carbide, Gallium Arsenide, Zinc Sulfide, Gallium phosphate, Cadmium sulfide etc.
Aluminium oxide, Silicon dioxide, aluminosilicate and zeolites:
One of the most significant industrial processes in which aluminium oxide, Al2O3, (also known as alumina) participates is platforming, in which naphtha is reformed over alumina impregnated with platinum or rhenium. Both the oxide and the metals fulfil catalytic functions, demonstrating bifunctional catalysis. On the surface of alumina, there exist, hydroxyl groups, which are negatively charged sites to which a hydrogen ion may be bonded and function as an acid catalyst.
Substituted acidic oxide used in industry is silicon dioxide (silica). It becomes especially active after being coated with an acid (such as phosphoric acid), which increases the number of active acidic sites. For example, ethanol is produced by hydrating ethene with silica that has been coated with phosphoric acid:
Aluminosilicate catalyst is strong for its acidic nature. the production of Silicon dioxide and aluminium oxide. The silicon ions Sio4 4- contained group in tetrahedral sites. If aluminium ions overlapped with silicon ions so it's called Aluminosilicate.

Zeolites are a kind of aluminosilicate that has sparked a lot of curiosity in recent years. Because of the various ways in which the atoms can be organised, there exist several zeolites. Their silicate and aluminate ion topologies can contain vast free spaces in three-dimensional structures that allow for cations like sodium and calcium, as well as molecules like water. In various zeolites, the gaps are linked and create lengthy channels and pores of varying widths. ZSM-5, a zeolite extensively employed in many catalytic processes, is made from sodium aluminate (an aluminium oxide solution in aqueous sodium hydroxide) and a colloidal solution of silica, sodium hydroxide, sulfuric acid, and tetrapropyl ammonium bromide.
It is a very good catalyst for the conversion of methylbenzene (toluene) to the three dimethyl benzenes, for example (xylenes). Unfortunately, the mixture generated only includes approximately 25% 1,4-dimethyl benzene (p-xylene), the isomer required for the production of polyesters, and the remainder, 1,2- (o-xylene) and 1,3-dimethyl benzenes (m-xylene), is not desired in such high amounts.
If the zeolite is washed with phosphoric acid and heated rapidly, however, minute particles of phosphorus(V) oxide are formed on the surface, making the pores somewhat narrower. This inhibits the diffusion of the 1,2- and 1,3-isomers, which are trapped in the pores until they are transformed to the 1,4-isomer. Because of this extraordinary selectivity, the yield of the 1,4-isomer may be enhanced from 25% to 97.5%.
The zeolite's capacity to adsorb some molecules while rejecting others allows it to function as a molecular filter. For example, in the production of ethanol from ethene or biomass, an aqueous solution of ethanol is created, with 4 per cent water remaining even after several distillations. Further filtration of ethanol necessitates the use of a zeolite, which preferentially absorbs water.
Table 2: Industrially processed zeolites
References:
- Chinchen, G. C., K. Mansfield, and M. S. Spencer. "The methanol synthesis: How does it work." CHEMTECH;(USA) 20.11 (1990).
- Lovelock, Sarah L., et al. "The road to fully programmable protein catalysis." Nature 606.7912 (2022): 49-58.
- Esterhuizen, Jacques A., Bryan R. Goldsmith, and Suljo Linic. "Interpretable machine learning for knowledge generation in heterogeneous catalysis." Nature Catalysis 5.3 (2022): 175-184.
- Fang, Siyuan, and Yun Hang Hu. "Thermo-photo catalysis: a whole greater than the sum of its parts." Chemical Society Reviews (2022).
- Bortolato, Tommaso, et al. "The advent and development of organophotoredox catalysis." Chemical Communications 58.9 (2022): 1263-1283.
- Guan, Yani, et al. "Machine learning in solid heterogeneous catalysis: Recent developments, challenges and perspectives." Chemical Engineering Science 248 (2022): 117224.
- Cheng, Xu, et al. "Recent Applications of Homogeneous Catalysis in Electrochemical Organic Synthesis." CCS Chemistry 4.4 (2022): 1120-1152.
- Wang, Xiangyu, et al. "Zeolite nanosheets for catalysis." Chemical Society Reviews (2022).
- Mukhtar, Ahmad, et al. "Current status and challenges in the heterogeneous catalysis for biodiesel production." Renewable and Sustainable Energy Reviews 157 (2022): 112012.
- Guo, He, et al. "Review on remediation of organic-contaminated soil by discharge plasma: Plasma types, impact factors, plasma-assisted catalysi
- s, and indexes for remediation." Chemical Engineering Journal (2022): 135239.
- Nandal, Neha, and Suman L. Jain. "A review on progress and perspective of molecular catalysis in photoelectrochemical reduction of CO2." Coordination Chemistry Reviews 451 (2022): 214271.
- Papanikolaou, Georgia, et al. "Catalysis for e-chemistry: need and gaps for a future de-fossilized chemical production, with focus on the role of complex (direct) syntheses by electrocatalysis." ACS catalysis 12.5 (2022): 2861-2876.
- Kumar, Parveen, et al. "Magnetically active iron oxide nanoparticles for catalysis of organic transformations: A review." Tetrahedron (2022): 132641.
- Wang, Chunyang, et al. "Versatile Titanates: Classification, Property, Preparation, and Sustainable Energy Catalysis." Advanced Functional Materials 32.5 (2022): 2108350.






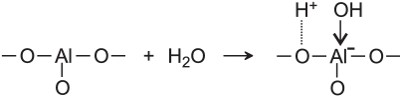






No comments:
Post a Comment
if you have any doubts, please let me know